Project Lead: Chad Curtis, PhD student, Chemical Engineering Department
eScience Liaison: Ariel Rokem
One highly underrepresented, but critically needed, area of study is inflammation-mediated central nervous system (CNS) disease. Inflammation in the CNS, mediated by activated microglia and astrocytes, is implicated in the development of several neurologic disorders in both children and adults. Strategies to target microglia/astrocytes and treat neuroinflammation could not only slow disease progression, but also promote repair and regeneration, enabling normal development and maturation of the brain. Hence, there is a crucial need for development of tailorable therapeutic platforms targeting neuroinflammation for the treatment and management of neurological disorders. However, therapeutic delivery to the brain also presents a challenge on multiple levels due to the presence of the blood-brain barrier (BBB), the diffuse nature of most brain diseases, and the brain microenvironment, of which a therapeutic must navigate to reach a target site.
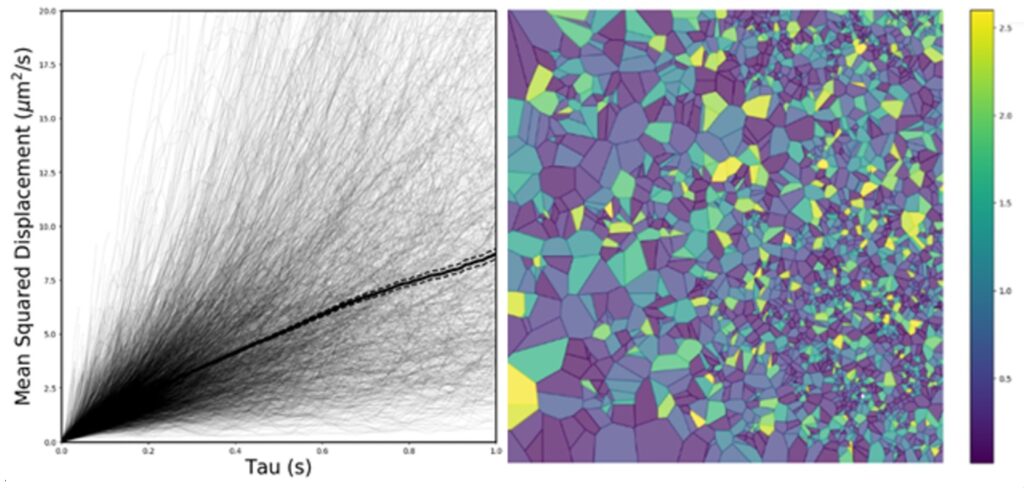
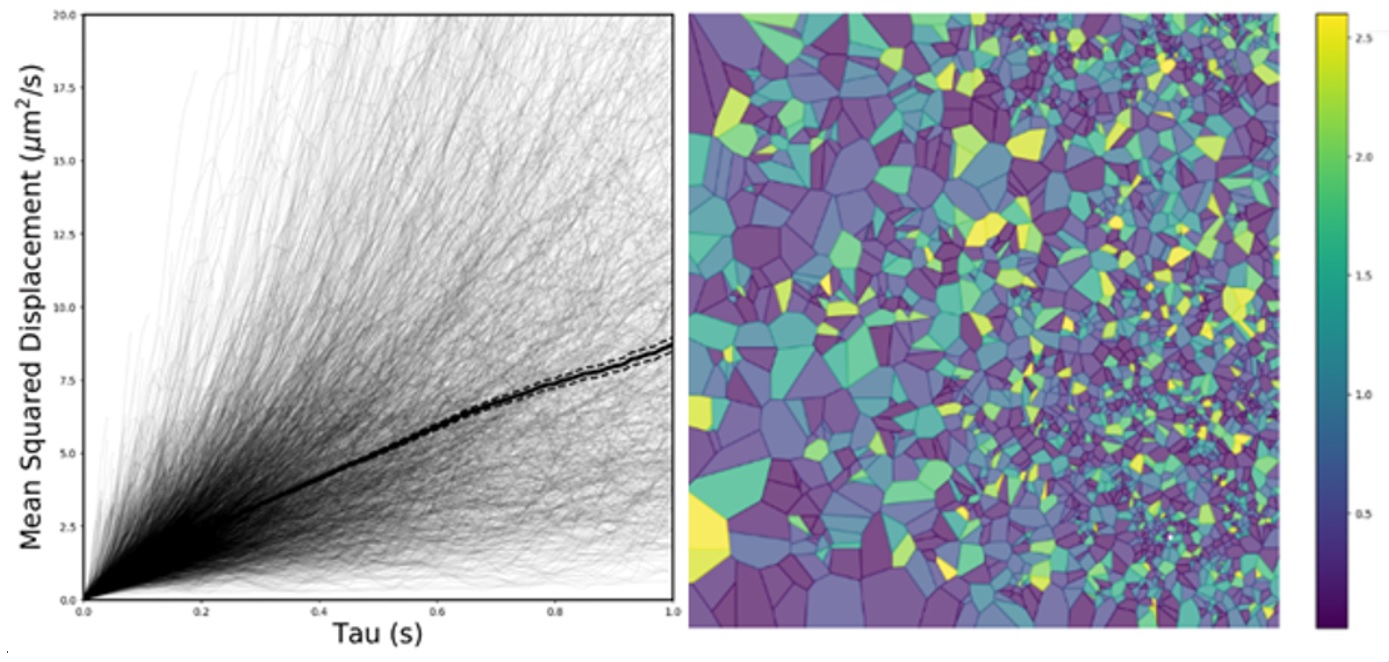
The ways in which changes to the extracellular matrix, brain oedema, glial cell function, and BBB disruption affect the diffusion, interactions, and cellular uptake of therapeutics following injury to the brain represents a current major knowledge gap. Nanotechnology, which consists of small, highly-tailorable platforms, can provide a modality to survey the disease environment,7-10 providing information through analysis of nanoparticle behavior and compartmentalization to fill this knowledge gap. Nanoparticle diffusion in the brain is subject to the geometry of the extracellular space (ECS) and is influenced by interaction with cells and proteins or through encountering local fluids flows within the brain microenvironment.
The overarching objective of our research proposal is to use data science tools to extract tissue-structure function from nanoparticle diffusion data obtained in the living brain. We utilize multiple particle tracking in a highthroughput, quantitative, real-time organotypic brain slice platform that captures the complexity of the in vivo tissue environment. In developing this technology, we aim to create both database and data analysis tools to extract robust data for more-in depth understanding of the tissue-specific structure-function relationships, particularly in the context of disease. We hypothesize that understanding mechanisms of nanoparticle behavior and compartmentalization can elucidate changes in the microenvironment in the brain in the presence of injury, specifically inflammation. We will start with analyzing data we have collected in the normal healthy developing brain to establish regional based differences in diffusion that can be correlated to known anatomical and microenvironmental constraints, and to current diffusion weight MRI (DWI) data in humans.